Highlights of our Work
2024 | 2023 | 2022 | 2021 | 2020 | 2019 | 2018 | 2017 | 2016 | 2015 | 2014 | 2013 | 2012 | 2011 | 2010 | 2009 | 2008 | 2007 | 2006 | 2005 | 2004 | 2003 | 2002 | 2001
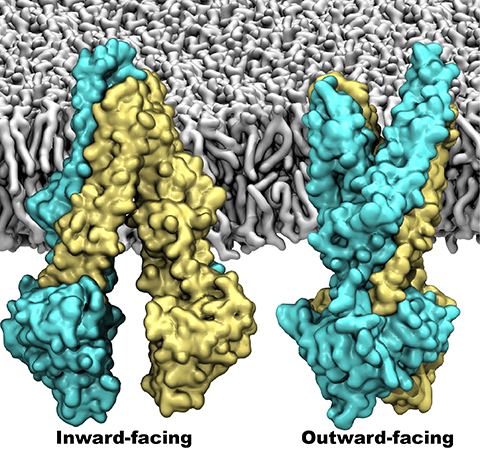
image size:
358.3KB
made with VMD
One of the most common mechanisms by which cancer and microbial cells develop resistance
against chemotherapeutic agents is to express a large number of specialized transporter proteins
in their cellular membrane that use the universal cellular energy of ATP to actively pump the drug
molecules to the outside. P-glycoprotein, a prominent member of such molecular "vacuum cleaners" and
responsible for multidrug resistance (MDR) in a wide variety of cancer types, accomplishes its role by
undergoing large-scale structural transitions in the cellular membrane through
which it effectively moves drug molecules from one side of the membrane to the other.
In a recent collaborative publication in Nature
with leading experimental groups
at Vanderbilt and Virginia, and employing advanced molecular modeling and
simulation techniques implemented in NAMD, a robust structural model was developed for
the unknown outward-facing state of P-glycoprotein,
allowing a full structural description of the transport cycle, and a novel mode of energy transduction.
Further details of the study can be found here.



