Mechanosensing
Cells explore their environment by sensing and responding to mechanical forces. Many fundamental cellular processes, such as cell migration, differentiation, and homeostasis, take advantage of this sensing mechanism. At molecular level mechanosensing is mainly driven by mechanically active proteins. These proteins are able to sense and respond to forces by, e.g., undergoing conformational changes, exposing cryptic binding sites, or even by becoming more tightly bound to one another. In humans, defective responses to forces are known to cause a plethora of pathological conditions, including cardiac failure, pulmonary injury and are also linked to cancer. Microorganisms also take advantage of mechano-active proteins and proteins complexes. Employing single-molecule force spectroscopy with an atomic force microscope (AFM) and steered molecular dynamics (SMD) simulations we have investigated force propagation pathways through a mechanically active protein complexes.Spotlight: Tight Job in the Gut (Feb 2015)
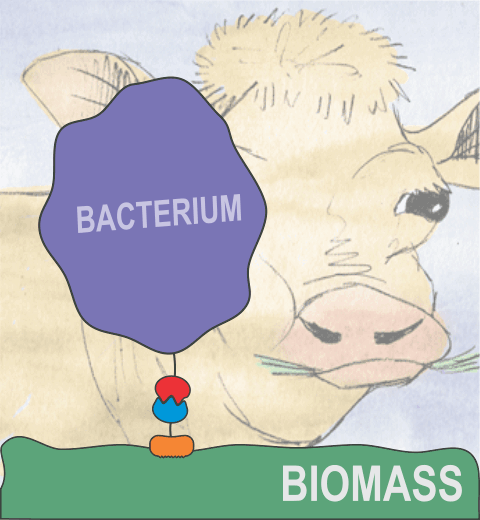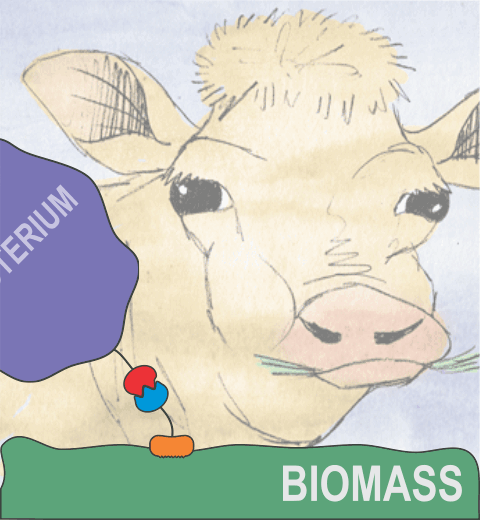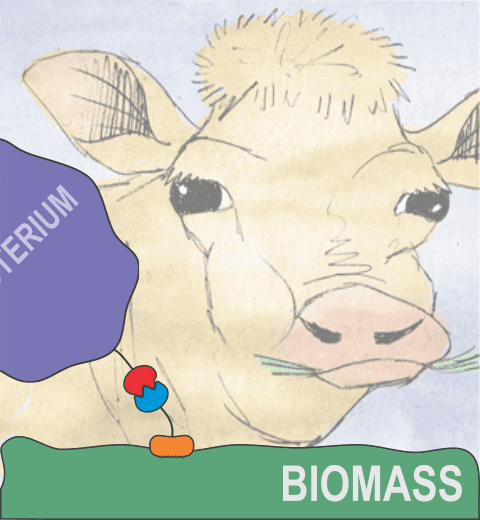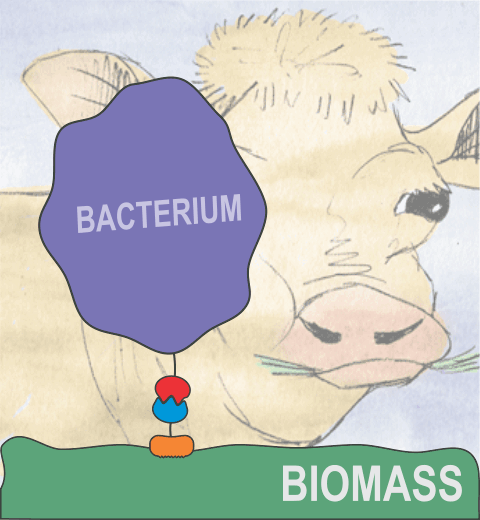
Bacteria can make a living from a very wide range of food sources. This ability makes them, for example, essential symbionts in animal digestive tracts where they assist their hosts in breaking cellulose fibers up into compounds degradable by the animal metabolism. Today, human gut bacteria, part of the human microbiome, are one of the hottest research topics in medicine. Gut bacteria face a particularly tough job in the rumen of the cow where they digest hardy cellulose fibers of grasses. Key to the job, taking place in a constantly moving fluid, are molecular tentacles, so-called cellulosomes, on the surface of the symbiotic bacteria. The cellulosomes develop a tight grasp on and then effective cleavage of cellulose. In a joint experimental-computational study researchers have investigated how in case of the bacterium Ruminococcus flavefaciens cellulosomes are built in a modular way, with molecular modules easily binding and unbinding during cellulosome construction, but sticking extremely strongly together during cellulosome digestive activity. As reported recently, single molecule force microscopy and molecular dynamics simulations using NAMD could show that under strain the adhesive bonds between cellulosome modules become stronger than seen in any other biomolecular system, in fact, become nearly as tight as strong chemical bonds. While the experimental data revealed bond strength and other characteristics, simulations reproducing the observed data provided a detailed view of the adhesive bond at atomic resolution, thereby revealing the physical mechanism underlying the uniquely adhesive property of cellulosomes. Gut bacteria and cellulosomes can be employed in 2nd generation biofuel generation (see highlight Waste into Fuel). More on gut bacteria and cellulosomes on our biofuels website.