Molecular Motors
Many cellular processes are driven by molecular motors, specialized proteins that utilize the energy generated from chemical reactions to perform physical work. Molecular motors play key roles in, for example, muscle contraction, protein degradation and recycling, cargo transport, and cell motility. Defects in motor function are implicated broadly in cancer, as well as numerous cardiovascular, neurological, and reproductive diseases. Researchers in the Theoretical and Computational Biophysics Group are interested in studying the complex conformational transitions that underlie the chemo-mechanical action of molecular motors toward characterizing their mechanisms and relationships to human disease.
Spotlight: Overcoming the Challenges of High-Resolution Data (Sep 2016)
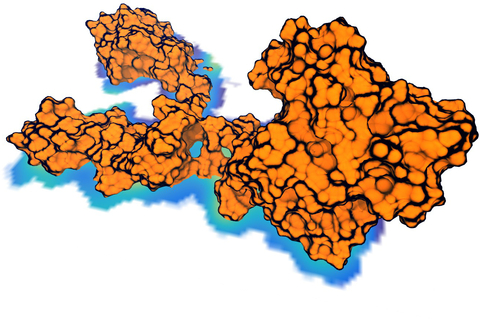
image size:
3.3MB
made with VMD
Living cells are brimming with the activity of macromolecular complexes carrying out their assigned tasks. Structures of these complexes can be resolved with cryo-electron microscopy (cryo-EM), wherein the complexes are first freeze-shocked into states characterizing their action and subsequently imaged by detection cameras. Recent advances in direct detection camera technology enable today's cryo-EM laboratories to image the macromolecular complexes at high-resolution, giving us a better view of the cell than ever before. Computational techniques like molecular dynamics flexible fitting (MDFF) are a key tool for producing atomic models of the imaged molecules, providing greater insight into their structure and function. The increased resolution of EM maps, which contain sharp valleys capable of trapping structures, presents a challenge to MDFF which was originally developed for maps in a lower resolution range. However, a recent study unveils two new techniques called cascade (cMDFF) and resolution exchange (ReMDFF) molecular dynamics flexible fitting to overcome the hurdles posed by high-resolution maps. The refinement is achieved by interpreting a range of cryo-EM images, starting with an image of fuzzy resolution and progressively improving the image's contrast until near-atomic resolution is reached. These techniques were employed to solve the structure of the proteasome, the recycling machine of the human cell. New analysis schemes that look at the flexibility of the obtained structure provide a measure of model uncertainty within the near-atomic EM images, improving their contrast. All the tools are available on cloud computing platforms allowing community-wide usage at low monetary cost; the complex computations can now be performed at the cost of a cup of coffee.



