Research Topics - Steered/Interactive Molecular Dynamics
Knowledge of the mechanism of association, dissociation and unfolding of macromolecules is important for many biological processes. Among the examples are the binding and dissociation of substrates of enzyme reactions, the recognition of ligands by their receptors or the elastic resopnse of mechanical proteins. In order to study such processes external forces can be applied reducing energy barriers and therefore increasing the probability of unlikely events on the time scale of molecular dynamics. This approach has the advantage that it corresponds closely to micromanipulation through atomic force microscopy or optical tweezers. The external force techniques can be applied to study many processes, including dissociation of avidin-biotin complex, dissociation of retinal from bacteriorhodopsin, stretching of titin, etc. The molecular dynamics program NAMD, developed in the group, is capable of performing several different kinds of SMD, including rotation or translation of one or more atoms. The group's molecular graphics program VMD provides a powerful means of visualizing these simulations, and through the Interactive Molecular Dynamics (IMD) interface can even allow SMD simulations to be performed in real time.
Papers
Onset of anthrax toxin pore formation. Mu Gao and Klaus Schulten. Biophysical Journal, 90:3267-3279, 2006.
What makes an aquaporin a glycerol channel: A comparative study of AqpZ and GlpF. Yi Wang, Klaus Schulten, and Emad Tajkhorshid. Structure, 13:1107-1118, 2005.
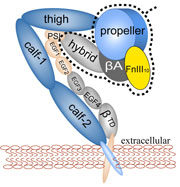
In search of the hair-cell gating spring: Elastic properties of ankyrin and cadherin repeats. Marcos Sotomayor, David P. Corey, and Klaus Schulten. Structure, 13:669-682, 2005.
Calculating potentials of mean force from steered molecular dynamics simulations. Sanghyun Park and Klaus Schulten. Journal of Chemical Physics, 120:5946-5961, 2004.
Insights into the molecular mechanism of rotation in the Fo sector of ATP synthase. Aleksij Aksimentiev, Ilya A. Balabin, Robert H. Fillingame, and Klaus Schulten. Biophysical Journal, 86:1332-1344, 2004.
Mechanisms of selectivity in channels and enzymes studied with interactive molecular dynamics. Paul Grayson, Emad Tajkhorshid, and Klaus Schulten. Biophysical Journal, 85:36-48, 2003.
Identifying unfolding intermediates of FN-III10 by steered molecular dynamics. Mu Gao, David Craig, Viola Vogel, and Klaus Schulten. Journal of Molecular Biology, 323:939-950, 2002.
Structural determinants of MscL gating studied by molecular dynamics simulations. Justin Gullingsrud, Dorina Kosztin, and Klaus Schulten. Biophysical Journal, 80:2074-2081, 2001.
Unfolding of titin immunoglobulin domains by steered molecular dynamics simulation. Hui Lu, Barry Isralewitz, André Krammer, Viola Vogel, and Klaus Schulten. Biophysical Journal, 75:662-671, 1998.
Molecular dynamics study of unbinding of the avidin-biotin complex. Sergei Izrailev, Sergey Stepaniants, Manel Balsera, Yoshi Oono, and Klaus Schulten. Biophysical Journal, 72:1568-1581, 1997.
Research Projects
- Mechanical Strength of the Titin/Telethonin Complex
- IMD and the Glycerol Channel
- Unbinding of Retinoic Acid from its Receptor
- Retinal's Binding Pathway in bR
- Unbinding of the Avidin-Biotin Complex
- Molecular Basis for Anthrax Intoxication
- Molecular Motor Scooting along DNA
- Lactose permease
- Bacteria Swim and Tumble
- Hook and Sensor of Cells
- Computational Force Microscopy
- Detecting DNA Orientation with a Nanopore